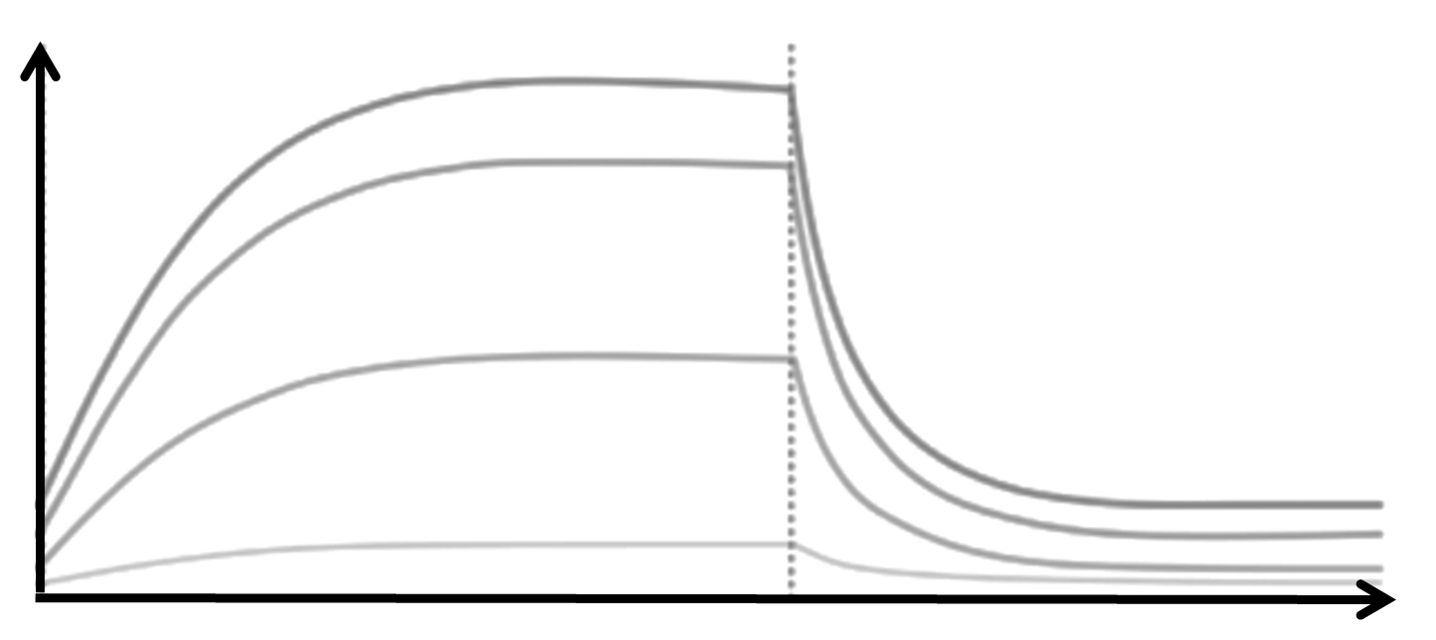
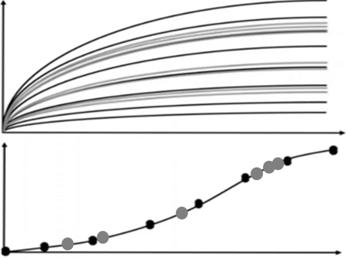
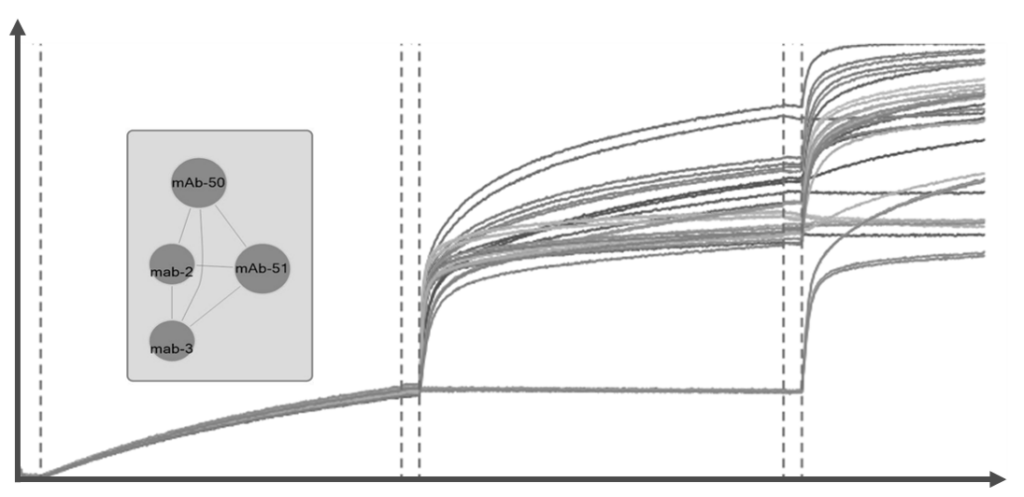
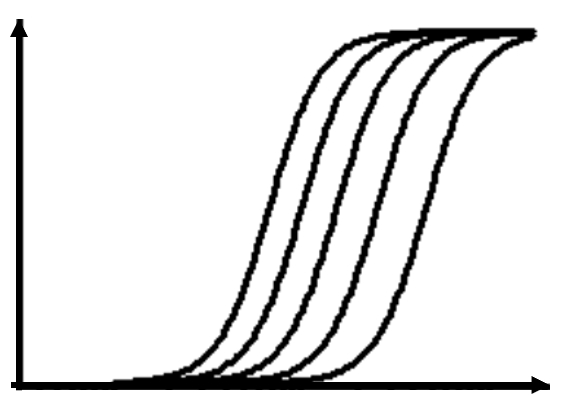
Successful IND submission and market authorization must rely on efficient combination of drug discovery and preclinical processess.
The development of drugs is an expensive and lengthy process. During discovery and preclinical phases, researches must screen and select multiple hit and lead candidates, optimize functional and biophysical properties, investigate developability and define optimal conditions for production and manufacturing. Implementing binding assay in the early stages of drug discovery can decrease downstream failure rates and prevent unnecessary spending of time and resources.
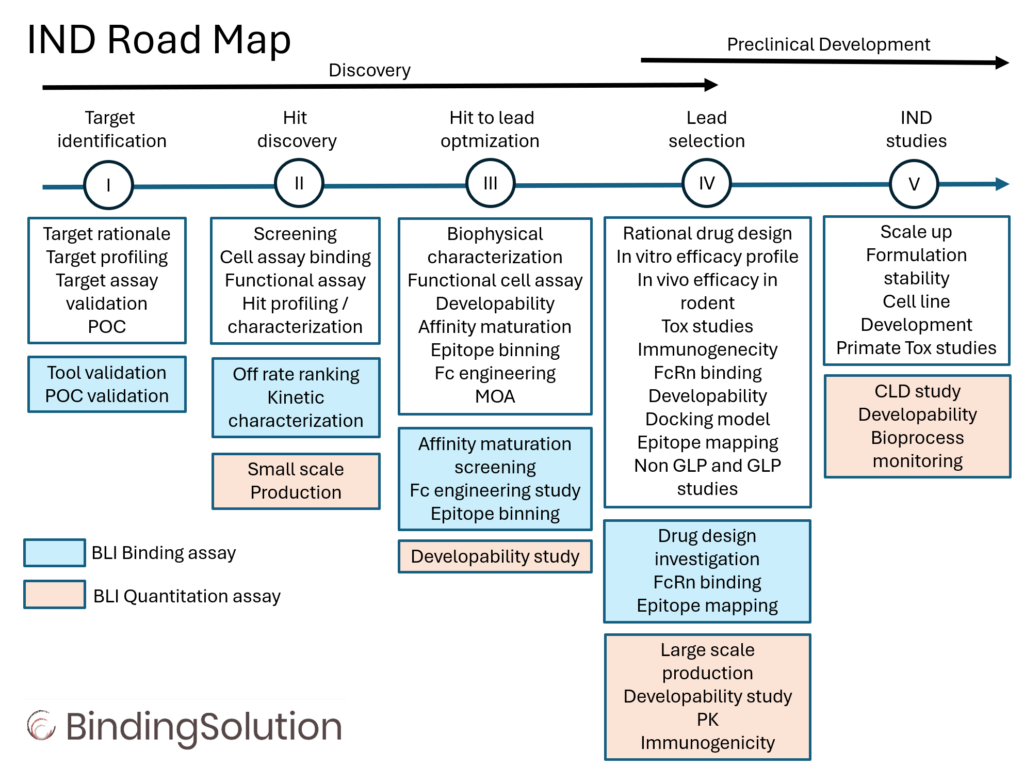
IgG Drug Discovery and Preclinical Workflow
BLI can support diverse molecular binding applications ranging from library screening assays (e.g Koff ranking, functional screening), drug lead candidate selection and characterization (e.g kinetic & affinity measurement, epitope binning), structure-activity relationship studies (e.g epitope mapping…), cell line development (CLD) monitoring during biologic production process, and optimization and surveillance of bioprocess during developability studies and development phases (EC50 dose response).
Besides applications with regard to therapeutic discovery and development, the versatility of BLI can be used to monitor target expression processes optimization and validate protein functionality thoughout production, purification and formulation steps. Consequently, whether soluble target or embedded target (e.g liposome, VLP, viral vector, nanodisc…), kinetic analysis with binding partners and quantitation assays during production steps can be perfomed to control the bioproducts quality.
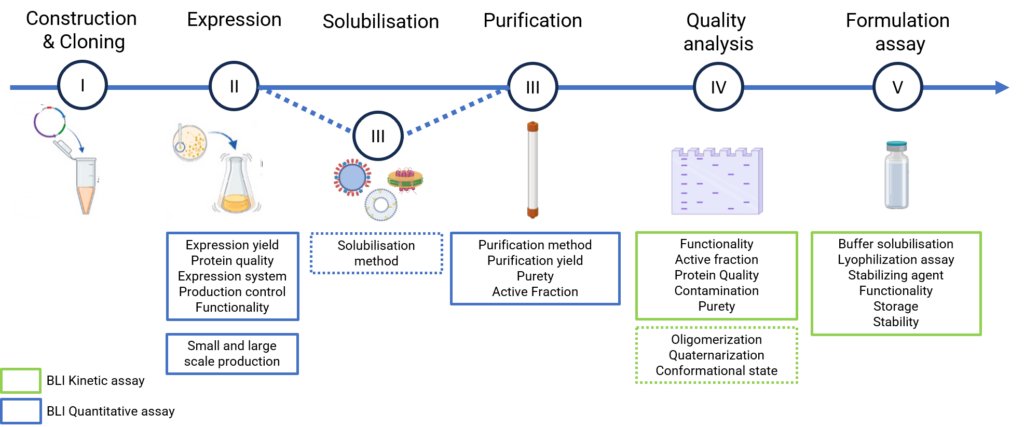
Target Production Workflow and BLI Monitoring
Octet® BLI instrument provides unmatched ease-of-use capability for multiple research applications and assay development from high throughput screening and quantitation campaigns to high resolution kinetic characterizations.
The robustness and versatility of Octet® BLI platform empowers discovery and preclinal development processes. It enables to save both time and resources through accelerating time to market, speeding up project completion, increasing project capacity, derisking program delays, and preventing late-stage failures.
BindingSolution offers robust BLI binding analytical service platform to provide detailed insights into your product’s biological interactions.
BindingSolution’s expertise can help you to streamline and empower your drug discovery and development processes.
Our BLI Analytical Service Platform at a Glance
→Affinity & kinetic measurement (KD, Kon and Koff)
→Hit/lead or mutants binding analysis (e.g affinity maturation, Tm improvement, germlining, PTM removal, humanization …)
→Allosteric effect on Protein binding assay (e.g cofactor…)
→Biologic functionality after labelling / coupling / fusion (e.g ADC, biotin), reformating (e.g Fc fused, bispecific constructs …) or Fc engineering (e.g S297N, afucosylation…)
→Independently or simultaneously multispecific binding assay (e.g DART, bite, TCE, diabody…)
→ Effector function modulation through Binding analysis to FcgR and FcRn at pH 6.0
→QC and functionality of soluble targets (e.g ECD, peptide mimetic, enzyme, aptamer, IgG, small molecule…) or protein embedded in lipid particles (e.g nanodisc, proteoliposome, VLP…)
→Off rate protein screening from hybridoma / bacteria / cell culture supernatant (e.g Affinity maturation campaign)
→Off rate pH dependent screening assay (e.g sweeping Antibody, acidic pH dependent Antibody…)
→Early epitope screening assay to select binders specific to a specific or new epitope
→Single concentration screening assay to assess KD (e.g epitope / paratope mapping, alanine scanning, affinity maturation, PTM removal, …)
→Bioproduct (e.g Fab, scFv, IgG, bispecific Antibody…) and target monitoring from crude extract, culture supernatant, hybridoma, cell lysate
→Bioproduct quantitation from cell line development supernatant (e.g hormone, multispecific Antibody, …)
→Serum humoral response dosing from biological samples after immunization, disease, infection or vaccination
→Virus vector titration (e.g AAV…) and embedded target into lipid particle quantitation
Cross-Competition Epitope Binning and Epitope Targeting
→Competitive Binning assay between antibodies to identify if IgG target similar or different epitopes
→Epitope Binning between IgG and benchmarked protein to determine if the recognized epitopes overlap
→Early epitope binding assay from crude supernatant to select binders against sought epitope (ligand binding domain, dimerization domain…)
→Early epitope binding assay to identity binders capable of targeting a wide range of epitopes
→EC50 determination for functional sample activity analysis during developability studies and bioprocesses (e.g thermal stress, storage assay, lot variability, stability, production, formulation…)
→EC50 determination during allosteric conformational change (e.g ATP…)
→EC50 determination of targets after purification steps (e.g storage, precipitation, stability, purety, contamination…)