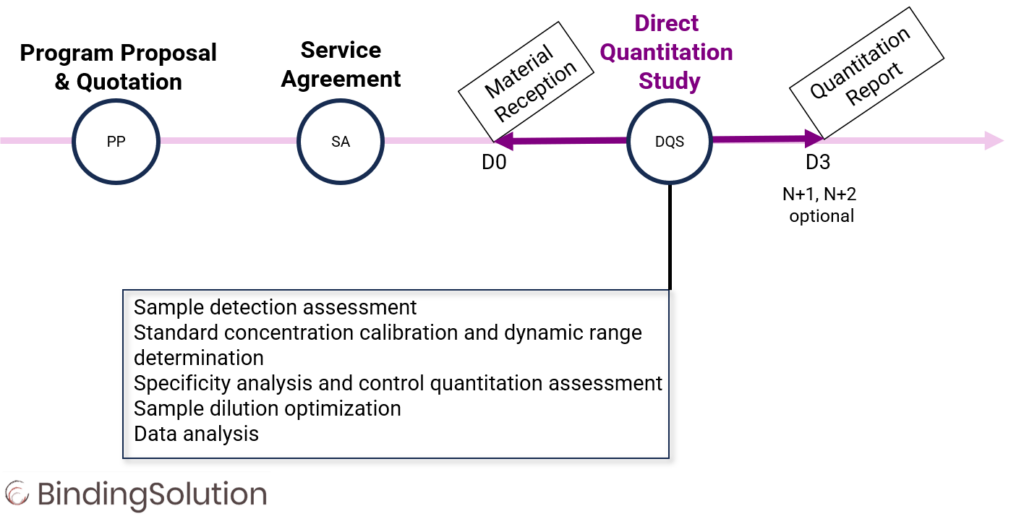
Integrity, Purity, Quality and Quantity of Biologics are Key Attributes that can be Monitored Throughout the Discovery and Bioprocess Workflow

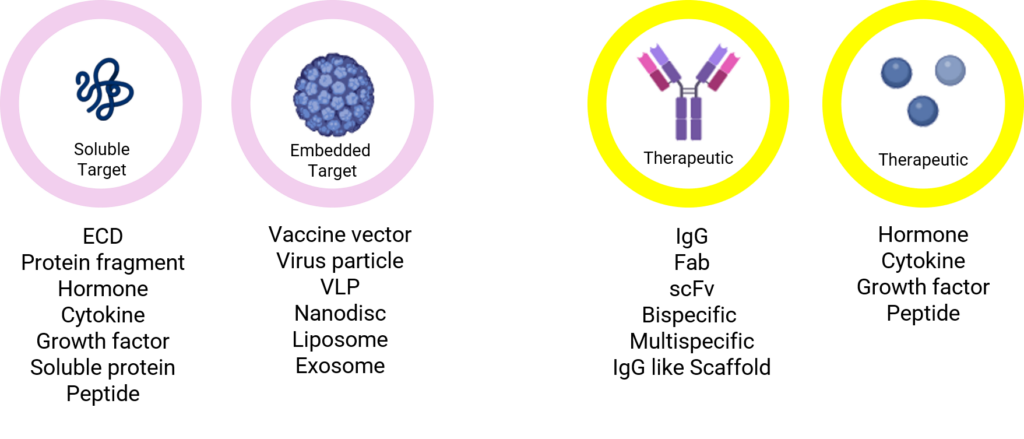
Analyte Diversity for BLI Quantitation
Analytical techniques that measure protein quantity and quality are used in nearly all stages of IND process, from early discovery stage to manufacturing. The high cost and lengthy times associated with these quality control steps have forced pharmaceutical companies to develop high sensitive strategies that give decisive guidance and streamline the overall workflow.
Octet® BLI high throughput Dip and Read quantitation assay allow titration of several hundred of bioproducts (e.g target, therapeutic, viral particle…) from crude samples such as supernant hybridoma or host cell without the need of filtration or purification. According to the assay, BLI quantitation detection range can be set-up for quantity down to 1-10 ng/ml.
The monitoring of Hybridoma and host cell culture production yield enables to exclude poor protein expressing clones during lead candidates discovery phase, while the determination of proteins titer is critical to identify high-producing clones during cell line development (CLD). The same principle can be applied to monitoring production yield during fermentation in a bioprocess optimization, and to characterize matrices with respect to dynamic binding capacity of affinity chromatographic columns in downstream process development.
BLI quantitation assay can be used to monitor soluble target expression processes both during production phases optimization and scale-up protein production. With a customized assay, target quantitation provides data on folding status and functionality.
Quantitation assay also enables the estimatation of the quantity of targets embedded in virus particle or diplay on nanoparticles.
Functional characterization and quality control of virus particles generation in gene therapy or vaccin development are other examples where particle titrations are essential to monitor capsid integrity and production yield throughout the bioprocess workflow.
Looking beyond simple quantitation assay, BLI quantification methods can be used for a plethore of specific applications such as assessing bioproduct or target quality with respect to post-translational modifications, dosing protein A contamination, monitoring IgG humoral response after immunization campaign and vaccination or titering drug candidate during PK or PD studies.
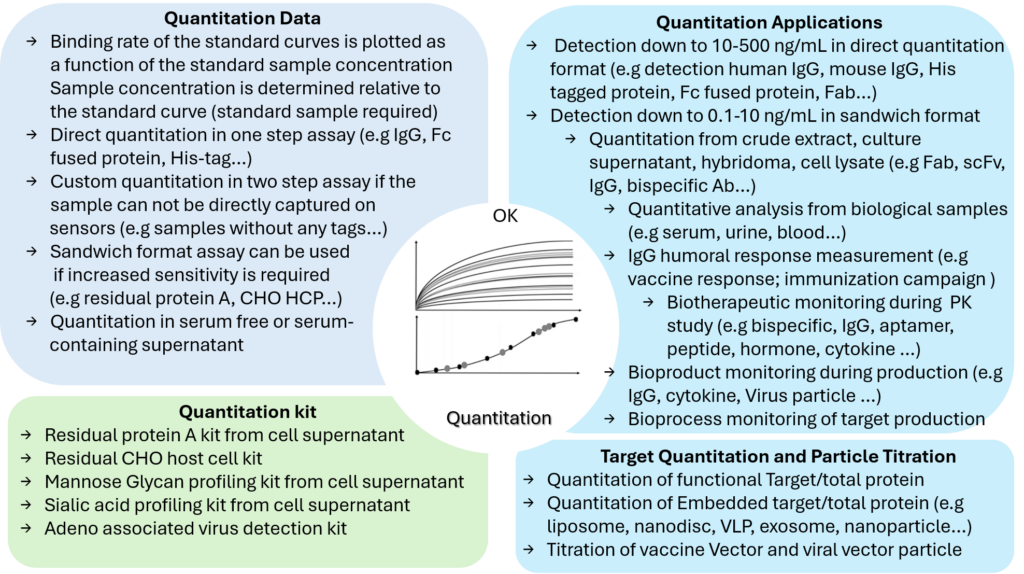
Insight of Samples Quantitation Applications by BLI
Octet® BLI system and Dip and Read biosensor architectures are readily applied in crude samples quantitation, unlike other fluidic-based biosensor detection SPR platforms. Besides label-free BLI quantitation platform combines high throughput analysis (several hundred of sample in one-step assay), low samples consumption, non-destructive and recoverable samples approaches.
BindingSolution offers versatile BLI binding measurement service platform to provide detailed insights into your product’s biological interactions.
BindingSolution’s expertise can help you to streamline and empower your drug discovery and development processes.
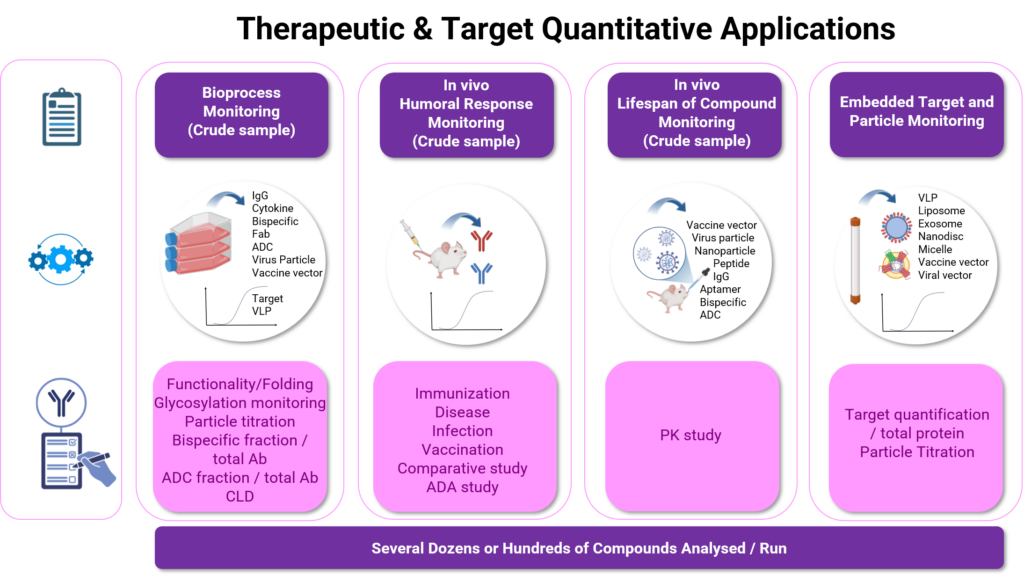
Our Analytical Quantitation Service Platform at a Glance