Potency EC50 Dose Response Assay Enables to Determine the Active Fraction of the Biologic in the Sample and Provides Direct Information on its Efficiency

To determine how much compound is present under an active form in a sample, a relative dose response bioassay can rapidly assess the EC50 and determine functional activity of the sample compared to a standard data set. Common applications include the characterization of antibodies, vaccines, therapeutic proteins throughout developability studies, manufacturing, stability testing or lot release.
Octet® BLI instrument is an easy to use analytical platform that can monitor biologics functional activity at early and late stage of drug development process. Direct, label-free potency dose effect assay appears as an alternative approach to KD determination, correlating binding to activity and substantially reducing assay development time compared to EC50 ELISA endpoint assays.
During drug development process and manufacturing, BLI EC50 dose response assays helps to provide assurance of the quality, stability, efficacy and consistency of the sample (e.g CLD, batch release…).

Therapeutic Candidate Diversity
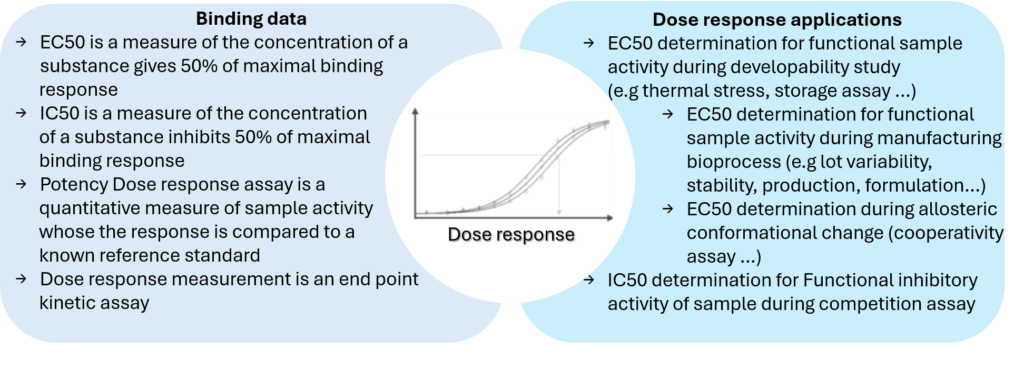
BLI EC50 Dose Response Applications
BindingSolution offers versatile BLI binding measurement service platform to provide detailed insights into your product’s biological interactions.
BindingSolution’s expertise can help you to streamline and empower your drug discovery and development processes.
