Similar to SPR, BLI principle relies on slight changes in reflected light at a sensor surface upon formation of molecular complexes.
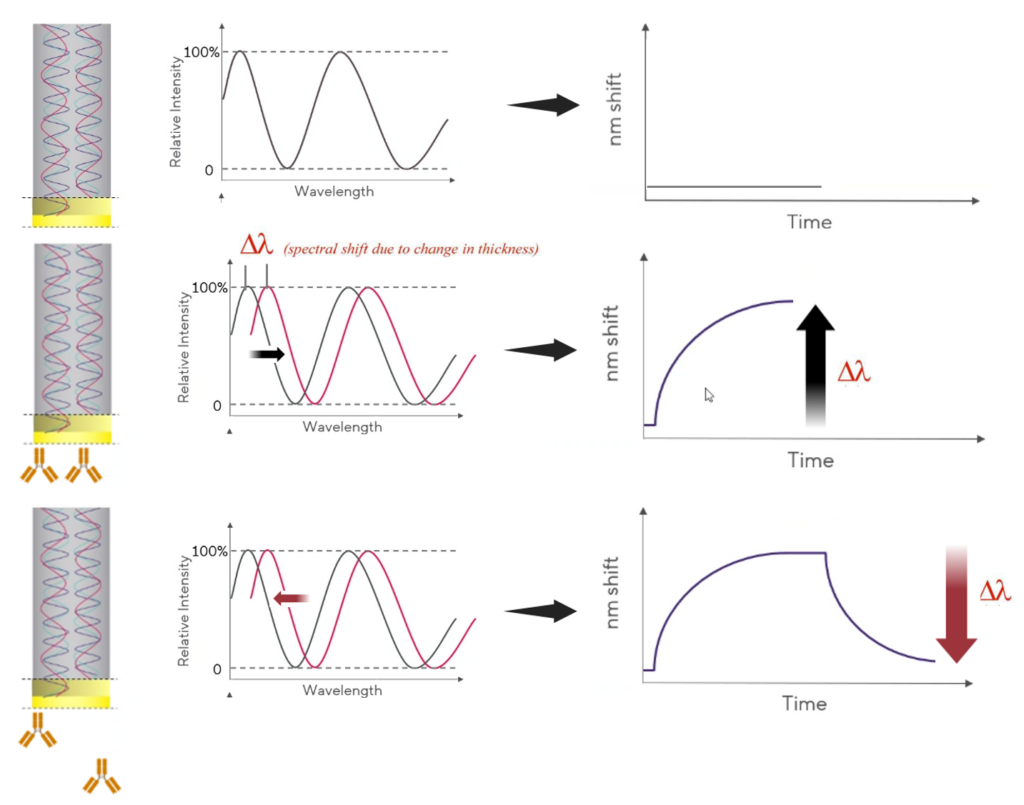
Bio-Layer Interferometry (BLI) is an optical analytical technique that analyzes the interference pattern of white light reflected from two surfaces: a layer of immobilized protein on the tip of the optical fiber and an internal reference layer inside the biosensor.
Any change in the number of molecules bound to the biosensor tip causes a shift in the interference pattern that can be measured in real-time. The binding between a ligand immobilized on the biosensor surface and an analyte in solution produces an increase in optical thickness and leads to different interference patterns that vary with the wavelengths of white light. The changes thickness is measured as a wavelength shift Δλ, which is directly proportional to the concentration of the analyte bound on the ligand. The wavelength shift is reported in real time and kinetics parameters such as association and dissociation rate constants and equilibrium dissociation constant are then calculated from sensorgram data.
In BLI, biomolecular interactions between two binding partners are converted into response signals in real-time allowing users to design methods that can help in rapidly optimizing binding assays of various biological molecules.
BLI technology has been developed to overcome issues related to SPR based analysis kinetic parameters of biding. In SPR, microfluidics is used to deliver samples to a biosensor surface with immobilized molecules of interest, whereas BLI uses dip-and-read approach where biosensor tips are immerged into the microtiter wells containing samples. Microfluidic channel clogging is a common SPR issue that requires regular machine maintenance. In addition, fluidics-free system in theory allows unlimited length of association and dissociation phases that in practice are only limited by evaporation. In contrast, SPR’s closed system is limited by the microfluidics volume.